Tekniker advances in the development of implants to regenerate peripheral nerve lesions
The Basque technology centre is developing implants that restore nerve functionality by combining advanced manufacturing technologies and biomaterials. Successful results obtained with animals have been published in the prestigious scientific journal ACS Biomaterials Science and Engineering.
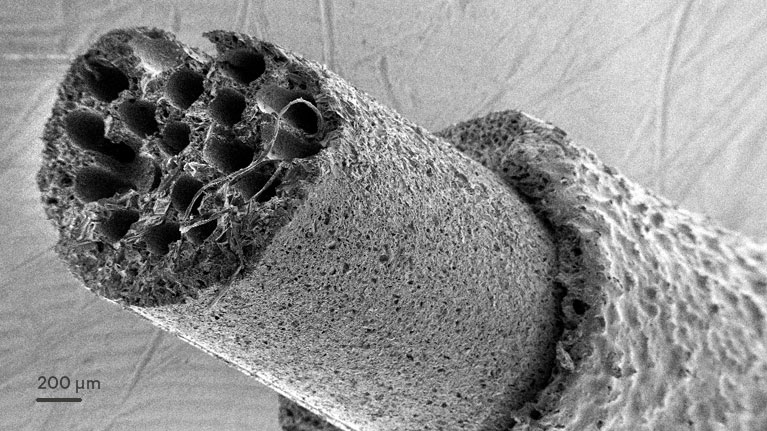
Lesions involving peripheral nerves usually caused by traumatic accidents have a severe incidence in terms of quality of life for patients and could produce motor control deficits and alterations in sensorial functions.
Thanks to regenerative medicine it is possible to operate on patients with injuries in their peripheral nervous system. Progress has been made in this field, for instance, by Tekniker, the Basque technology centre member of the Basque Research and Technology Alliance (BRTA), within the framework of the European NEURIMP project whose highly successful results in preclinical trials on animals have been published in the prestigious North American scientific journal ACS Biomaterials Science and Engineering.
The solution intends to propose an alternative to using autologous implants to treat trauma patients.
As an alternative to these implants, Tekniker researchers have combined state of the art biocompatible and biodegradable materials with scalable manufacturing technologies to develop advanced implants that can recover peripheral nerve functionality.
“We develop biomimetic conduits that act as a guide in the process of reconstruction and regeneration of discontinuities in an injured nerve”, explains Iban Quintana, a Tekniker researcher.
In addition to coordinating the project, the technology centre has focused its activities on developing integrated technologies in areas of specialisation such as surface engineering and advanced manufacturing to process polymers at a micrometric scale and optimise the tubular structure (made of up microchannels) of implants to mimic native nerve geometry.
The Basque technology centre has also worked with synthetic and natural polymers synthesised by other consortium partners and their combinations to adapt micromanufacturing technologies to the biophysical properties of new materials.
Validated successfully in animals
Both the feasibility and effectiveness of Tekniker’s innovative technology have been validated in preclinical trials on animals, specifically with rats, to perform sciatic nerve implant surgery and the results reported were better than those obtained with commercial implants.
“The publication of these advances by specialised media acknowledges the top-quality research currently under way at Tekniker and will provide the necessary driving force to develop solutions in the field of regenerative medicine”, explains Iban Quintana.
Tekniker, in fact, is now addressing how to design a sustainable manufacturing process for Nerve Guidance Conduits (NGCs) and provide training actions allowing professionals to use and implement advanced manufacturing activities based on biomaterials.
Tekniker’s developments lend continuity to the line of research that was opened in the European NEURIMP project and finished at the end of 2017 with a consortium made up by the National Paraplegics Hospital in Toledo, the Basque biopharmaceutical company Histocell, the British universities of Sheffield and Westminster, the Dutch consultants Oserve and the biomaterials firms Vornia Biomaterials (Ireland) and Contipro (Czech Republic).
This initiative, which forms part of the 7th EU Framework Programme for Research and Development, was also chosen as a successful case by the European Commission. Likewise, Tekniker is a Cervera centre of excellence in the priority technology: Advanced Materials (Red Surfera).
This project has an impact on SDGs 3: Health & Wellbeing and SDGs 9 – Industry, innovation and infrastructures and contributes towards the economic, social and environmental pillars of sustainable development and, ultimately, society at large.
This project has received funding from the European Community´s Seventh Framework Programme (FP7-NMP-2013-SME-7) under grant agreement no 604450.
